Changes in Physicochemical Properties and Volatile Compounds of Roselle (Hibiscus sabdariffa L.) Calyx during Different Drying Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Analysis
2.2. Volatile Compounds
2.3. Microstructure
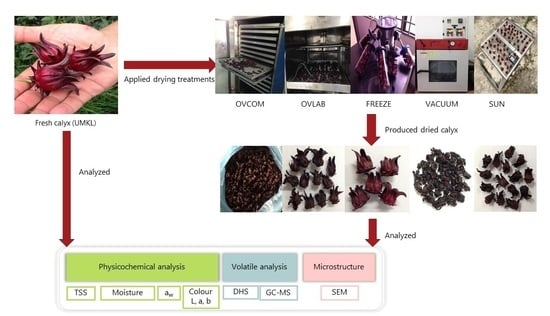
3. Materials and Methods
3.1. Sample Preparation
3.2. Drying Experiments
3.3. Physicochemical Analysis
3.4. Volatile Compounds
3.5. Microstructural Analysis
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mamat, M.R. 2 Villages of Roselle; Harian Metro: Kuala Lumpur, Malaysia, 2016; pp. 2–4. [Google Scholar]
- Husin, N.N. Roselle Plant Has High Demand for Export to Australia; Star Online Community: Petaling Jaya, Malaysia, 2010; pp. 10–12. [Google Scholar]
- Villani, T.; Juliani, H.R.; Simon, J.E.; Wu, Q.L. Hibiscus sabdariffa: Phytochemistry, Quality Control, and Health Properties. ACS Symp. Ser. 2013, 1127, 209–230. [Google Scholar] [CrossRef]
- Ibrahim, R.; Mazuki, N.A.F. The quality of roselle (Hibiscus sabdariffa L.) juices made from roselle calyces stored at different cold temperatures. Malays. Appl. Biol. 2013, 42, 67–71. [Google Scholar]
- Sabet Sarvestani, S.; Hosseini, S.M.; Farhangfar, S.H. Effect of aqueous-alcoholic extract of Hibiscus sabdariffa calyx and leaf calyx and leaf on performance, egg quality, immune system and antioxidant balance of laying hens. Iran. J. Appl. Anim. Sci. 2020, 10, 317–325. [Google Scholar]
- Shruthi, V.H.; Ramachandra, C.T. Roselle (Hibiscus sabdariffa L.) Calyces: A Potential Source of Natural Color and Its Health Benefits. Food Bioactives, 1st ed.; Apple Academic Press: Boca Raton, FL, USA, 2019; pp. 169–190. [Google Scholar] [CrossRef]
- Garg, H.; Kumar, R. Development in Solar Drying. In Proceedings of the 2nd Asian-Oceania Drying Conference (ADC 2001), Batu Feringhi, Pulau Pinang, Malaysia, 20–22 August 2001; pp. 297–319. [Google Scholar]
- Sulieman, A.M. Spray drying of karkade (Hibiscus sabdariffa L.) calyces and evaluation of the product. Int. J. Food Eng. 2014, 10, 157–165. [Google Scholar] [CrossRef]
- Builders, P.F.; Ezeobi, C.R.; Tarfa, F.D.; Builders, M.I. Assessment of the intrinsic and stability properties of the freeze-dried and formulated extract of Hibiscus sabdariffa Linn. (Malvaceae). African J. Pharm. Pharmacol. 2010, 4, 304–313. [Google Scholar]
- Meza-Jimenez, J.; Ramirez-Ruiz, J.; Diaz-Nunes, J. The design and proposal of a thermodynamic drying system for the dehydration of Roselle (Hibiscus Sabdariffa) and other agro-industrial products. African J. Agric. Res. 2008, 3, 477–485. [Google Scholar] [CrossRef]
- Daniel, D.L.; Huerta, B.B.; Sosa, I.A.; Mendoza, M.V. Effect of fixed bed drying on the retention of phenolic compounds, anthocyanins and antioxidant activity of roselle (Hibiscus sabdariffa L.). Ind. Crops Prod. 2012, 40, 268–276. [Google Scholar] [CrossRef]
- Zaman, H.U.; Das, P.; Das, P.; Sahu, N.K. Analysis of physicochemical, nutritional and antioxidant properties of fresh and dried Roselle (Hibiscus sabdariffa Linn.) calyces. Int. J. Pure App. Biosci 2017, 5, 261–267. [Google Scholar] [CrossRef]
- Gonzalez-Palomares, S.; Estarrón-Espinosa, M.; Gómez-Leyva, J.F.; Andrade-González, I. Effect of the temperature on the spray drying of Roselle extracts (Hibiscus sabdariffa L.). Plant Foods Hum. Nutr. 2009, 64, 62–67. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Kianmehr, M.H.; Samimi-Akhijahani, H. Influence of drying conditions on the effective moisture diffusivity, energy of activation and energy consumption during the thin-layer drying of berberis fruit (Berberidaceae). Energy Convers. Manag. 2008, 49, 2865–2871. [Google Scholar] [CrossRef]
- Lewicki, P.P. Design of hot air drying for better foods. Trends Food Sci. Technol. 2006, 17, 153–163. [Google Scholar] [CrossRef]
- Agudelo, C.; Barros, L.; Santos-Buelga, C.; Martínez-Navarrete, N.; Ferreira, I.C.F.R. Phytochemical content and antioxidant activity of grapefruit (Star Ruby): A comparison between fresh freeze-dried fruits and different powder formulations. LWT-Food Sci. Technol. 2017, 80, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Navarrete, N.; Salvador, A.; Oliva, C.; Camacho, M.M. Influence of biopolymenrs and freeze-drying shelf temperature on the quality of a mandarin snack. LWT 2019, 99, 57–61. [Google Scholar] [CrossRef]
- Fontana, A. Water Activity: Why it is Important for Food Safety? In Proceedings of the NSP International Conference on Food Safety, AlBuqerque, NM, USA, 16–18 November 1998; pp. 177–185. [Google Scholar]
- Decagon Device, I. Water Activity for Product Safety and Quality. Available online: http://www.aqualab.com/education/water-activity-for-product-safety-and-quality/ (accessed on 11 July 2017).
- Okanlawon, S.; Ibrahim, M.; Oyebanji, A. Effect of pre-drying treatment on the storage of dried tomatoes. Trop. Sci. 2002, 42, 40–41. [Google Scholar]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Juhari, N.H.; Lasekan, O.; Kharidah, M.; Ab Karim, S. Optimization of hot-air drying conditions on the physicochemical characteristics of torch ginger (Etlingera elatior). J. Food, Agric. Environ. 2012, 10, 64–72. [Google Scholar]
- Prachayawarakorn, S.; Prachayawasin, P.; Soponronnarit, S. Effective diffusivity and kinetics of urease inactivation and color change during processing of soybeans with superheated-steam fluidized bed. Dry. Technol. 2004, 22, 2095–2118. [Google Scholar] [CrossRef]
- Wu, H.Y.; Yang, K.M.; Chiang, P.Y. Roselle anthocyanins: Antioxidant properties and stability to heat and pH. Molecules 2018, 23, 1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, A.A.; Figiel, A. Comparison of traditional and novel drying techniques and its effect on quality of fruits, vegetables and aromatic herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodrigues, M.; Balaban, M.; Marshall, M.; Rouseff, R. Hot and cold water infusion aroma profiles of Hibiscus sabdariffa: Fresh compared with dried. J. Food Sci. 2011, 76, C212–C217. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Aroma compounds. In Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; pp. 340–402. [Google Scholar]
- Kroh, L.W. Caramelisation in food and beverages. Food Chem. 1994, 51, 373–379. [Google Scholar] [CrossRef]
- Chen, S.; Huang, T.C.; Ho, C.T.; Tsai, P.J. Extraction, analysis, and study on the volatiles in roselle tea. J. Agric. Food Chem. 1998, 8561, 1101–1105. [Google Scholar] [CrossRef]
- Juhari, N.H.; Varming, C.; Petersen, M.A. Analysis of Aroma Compounds of Roselle by Dynamic Headspace Sampling using Different Sample Preparation Methods. In XIV Weurman Flavour Research Symposium; Andrews, J., Taylor, D.S.M., Eds.; Context Products Ltd.: Cambridge, UK, 2014; pp. 87–90. [Google Scholar]
- Eichnerl, K.; Karel, M. The influence of water content and water activity on the sugar-amino browning reaction in model systems under various conditions. J. Agric. Food Chem. 1972, 20, 218–223. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y. Effects of pulsed-vacuum and ultrasound on the osmodehydration kinetics and microstructure of apples (Fuji). J. Food Eng. 2008, 85, 84–93. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y. Effect of pulsed vacuum and ultrasound osmopretreatments on glass transition temperature, texture, microstructure and calcium penetration of dried apples (Fuji). LWT-Food Sci. Technol. 2008, 41, 1575–1585. [Google Scholar] [CrossRef]
- Lewicki, P.P.; Pawlak, G. Effect of drying on microstructure of plant tissue. Dry. Technol. 2003, 21, 657–683. [Google Scholar] [CrossRef]
- Yousif, A.N.; Scaman, C.H.; Durance, T.D.; Girard, B. Flavor volatiles and physical properties of vacuum-microwave- and air-dried sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 1999, 4777–4781. [Google Scholar] [CrossRef] [PubMed]
- AOAC Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 1984.



| FRESH | VACUUM | FREEZE | OVCOM | OVLAB | SUN | Significance | |||
|---|---|---|---|---|---|---|---|---|---|
| Roselle calyx | Moisture content (%) | 89.4 ± 0.1 a | 12.9 ± 0.1 c | 9.6 ± 0.2 d | 9.5 ± 1.4 d | 8.9 ± 0 d | 15.6 ± 0.1 b | *** | |
| Water activity (Aw) | 1.000 ± 0 a | 0.605 ± 0 c | 0.414 ± 0 e | 0.482 ± 0 d | 0.395 ± 0 e | 0.727 ± 0 b | *** | ||
| Color value -measured in ground form | L* | 34.7 ± 0.4 b | 35.2 ± 1.1 b | 38.9 ± 0.3 a | 39.2 ± 0.4 a | 39.1 ± 0.4 a | 38.8 ± 0.5 a | *** | |
| a* | 4.5 ± 0.3 d | 4.5 ± 1.0 d | 11.8 ± 0.4 a | 10.5 ± 0.2 b | 7.2 ± 0.6 c | 4.8 ± 0.4 d | *** | ||
| b* | 5.9 ± 0 bc | 6.4 ± 0.5 b | 7.7 ± 0.1 a | 7.8 ± 0.2 a | 5.8 ± 0.1 c | 8.2 ± 0.5 a | *** | ||
| Filtrate | Total soluble solids (%) | 0.4 ± 0.1 f | 1.1 ± 0.1 e | 3.8 ± 0 a | 3.5 ± 0 b | 3.1 ± 0.2 c | 2.8 ± 0 d | *** | |
| Color value -measured in liquid form | L* | 43.1 ± 0 b | 45.0 ± 0.1 a | 35.6 ± 0.1 d | 35.5 ± 0.1 d | 35.6 ± 0.2 d | 36.2 ± 0 c | *** | |
| a* | 20.9 ± 0.2 a | 16.2 ± 0.2 b | 6.3 ± 0.1 e | 7.1 ± 0 d | 6.1 ± 0.1 e | 8.8 ± 0.1 c | *** | ||
| b* | 17.5 ± 0.2 a | 15.6 ± 0.1 b | 5.9 ± 0 d | 6.1 ± 0.1 d | 6.0 ± 0 d | 7.1 ± 0 c | *** | ||
| Compounds | Calculated RI a | Reference RI | ID b | Odor Description | FRESH | VACUUM | FREEZE | OVCOM | OVLAB | SUN | Significance g |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Terpenes | |||||||||||
| β-Myrcene | 1154 | 1170 | Standard | Musty, fruity, lemon, spice, woody d | 10534 | 989 | 542 | 0 | 683 | 0 | *** |
| Limonene | 1179 | 1200 | Standard | Citrus, fruity, green d | 39828 | 4057 | 9728 | 9807 | 11478 | 5197 | *** |
| 1,8-Cineole | 1187 | 1193 | Standard | Camphor, minty, pine, liquorice, mentholic d | 16625 | 869 | 421 | 1003 | 458 | 0 | *** |
| p-Cymene | 1256 | 1261 | Standard | Lemon, fruity, sweet, herbal d | 15767 | 2188 | 36723 | 34849 | 30681 | 4158 | * |
| α-Terpinolene | 1270 | 1297 | Standard | Woody, fruity, sweet, pine d | 11089 | 1227 | 4305 | 4218 | 4825 | 1415 | *** |
| Isoterpinolene | 1385 | 1331 | Literature | Woody, pine, citrus e | 4447 | 1104 | 920 | 6762 | 3073 | 4642 | *** |
| trans-Linalool oxide | 1426 | 1438 | Literature | Floral, creamy, earthy, green c | 321 | 5711 | 3316 | 8425 | 0 | 5514 | * |
| Neroloxide | 1460 | 1485 | Standard | Oily, flowery d | 1280 | 0 | 814 | 3038 | 1777 | 0 | * |
| Camphor | 1509 | 1498 | Literature | Camphor, green, leafy | 3211 | 1477 | 833 | 7072 | 3582 | 3502 | n.s. |
| Linalool | 1528 | 1534 | Standard | Citrus, herbal, flowery c | 548431 | 6071 | 5819 | 9672 | 17309 | 22827 | *** |
| α-isophorone | 1583 | 1591 | Literature | Woody, champor, musty e | 9145 | 376 | 2461 | 1185 | 0 | 1403 | *** |
| 1-p-menthen-9-al | 1602 | 1620 | Literature | Spice, herbal d | 647 | 3897 | 3007 | 7325 | 4224 | 7277 | n.s. |
| Aromadendrene | 1627 | 1628 | Standard | Sweet, dry d | 2063 | 0 | 976 | 0 | 2126 | 0 | n.s. |
| α-Bisabolene | 1653 | 1702 | Literature | Berry, spicy, citrus d | 7398 | 0 | 433 | 0 | 370 | 0 | *** |
| α-Terpineol | 1683 | 1682 | Literature | Pine, lillac, woody, floral e | 31865 | 2742 | 5374 | 7928 | 6826 | 10288 | *** |
| Azulene | 1724 | 1746 | Literature | Medicinal d | 35528 | 4193 | 2433 | 3614 | 28092 | 7563 | *** |
| δ-Cadinene | 1735 | 1749 | Literature | Thyme, medicinal, woody c | 15947 | 1516 | 1944 | 1798 | 2683 | 2979 | *** |
| α-Calacorene | 1896 | 1904 | Literature | Woody c | 1104 | 90 | 452 | 261 | 520 | 358 | *** |
| Esters Ethyl Ester | |||||||||||
| Ethyl acetate | 864 | 867 | Standard | Pineapple c, fruity d | 164108 | 168821 | 5750 | 63801 | 188847 | 48039 | n.s. |
| Acetate Esters | |||||||||||
| Methyl acetate | 807 | 810 | Literature | Fruity, solvent-like d | 14252 | 442728 | 13023 | 243853 | 132356 | 290587 | ** |
| 2-Methylpropyl acetate | 999 | 1017 | Standard | Fruity, flowery, strong, banana, pear d | 1631 | 17130 | 0 | 1005 | 1905 | 0 | *** |
| 3-Methylbutyl acetate | 1111 | 1112 | Literature | Sweet, banana, fruity, green e | 602 | 29269 | 715 | 1998 | 5342 | 21192 | ** |
| Pentyl acetate | 1165 | 1172 | Literature | Herbal d | 1834 | 1415 | 0 | 1772 | 658 | 8173 | n.s. |
| Hexyl acetate | 1266 | 1293 | Standard | Fruity, Herbal c | 1041 | 1140 | 0 | 0 | 0 | 13768 | * |
| Phenethyl acetate | 1797 | 1795 | Standard | Rose, floral, fruity, sweet d | 0 | 2889 | 0 | 0 | 0 | 17139 | ** |
| Other esters | |||||||||||
| Methyl 2-methylpropanoate | 907 | 910 | Literature | Fruity, floral e | 3557 | 10362 | 906 | 736 | 783 | 0 | *** |
| Methyl 2-methylbutanoate | 991 | 1000 | Literature | Apple, fruity c | 799 | 6133 | 0 | 0 | 0 | 0 | *** |
| Methyl 3-methylbutanoate | 1003 | 1011 | Literature | Fruity, apple d | 13640 | 4149 | 12 | 0 | 120 | 0 | *** |
| Methyl pentanoate | 1076 | 1086 | Literature | Sweet, ethereal, apple d | 1606 | 0 | 0 | 397 | 101 | 0 | * |
| Methyl hexanoate | 1176 | 1196 | Standard | Fruity, fresh, sweet c | 1515 | 1691 | 213 | 3057 | 662 | 17626 | * |
| 3-Methylbutyl butanoate | 1236 | 1256 | Literature | Fruity, apple, spicy, buttery e | 40922 | 0 | 0 | 1709 | 0 | 0 | *** |
| Methyl octanoate | 1375 | 1401 | Standard | Orange c | 0 | 299 | 174 | 586 | 466 | 4871 | ** |
| Methyl nonanoate | 1471 | 1481 | Literature | Coconut c | 0 | 0 | 87 | 847 | 377 | 1622 | *** |
| Methyl salicylate | 1759 | 1797 | Standard | Peppermint c | 602 | 447 | 176 | 1020 | 356 | 480 | n.s. |
| Aldehydes | |||||||||||
| 2-Methylpropanal | 787 | 789 | Literature | Green, pungent, burnt, malty d | 0 | 11900 | 5142 | 7050 | 12196 | 6909 | * |
| 2-Methylbutanal | 893 | 896 | Standard | Cocoa, almond, maltyc fermented f | 33135 | 22471 | 4677 | 13602 | 20546 | 8145 | * |
| 3-Methylbutanal | 900 | 917 | Standard | Fruity, almond, toasted, malty, green d | 14846 | 37241 | 5803 | 12035 | 29298 | 22709 | n.s. |
| Pentanal | 948 | 968 | Standard | Almond, malty, pungent d | 46310 | 8401 | 7752 | 22431 | 29236 | 20175 | n.s. |
| Hexanal | 1068 | 1087 | Standard | Grassy c | 100121 | 5753 | 16053 | 59056 | 27115 | 46659 | *** |
| Heptanal | 1172 | 1192 | Standard | Fatty, citrus, rancid c | 15731 | 946 | 1235 | 6669 | 3076 | 5293 | *** |
| 2-Hexenal | 1196 | 1205 | Literature | Apple, green, leaf c | 14741 | 0 | 3260 | 1753 | 2056 | 1767 | *** |
| Octanal | 1279 | 1311 | Standard | Orange peel, pungent f | 20491 | 772 | 1263 | 4275 | 2202 | 6123 | ** |
| Nonanal | 1379 | 1402 | Standard | Fatty, citrus, green c | 75446 | 3183 | 5419 | 23189 | 12695 | 20012 | *** |
| Decanal | 1480 | 1511 | Standard | Green, waxy, floral, tallow d | 5368 | 379 | 388 | 1733 | 0 | 2799 | * |
| Benzaldehyde | 1503 | 1537 | Standard | Almond c | 53396 | 22030 | 8172 | 11396 | 30133 | 49355 | * |
| Phenylethanal | 1622 | 1636 | Literature | Honey, sweet c | 2733 | 3822 | 1344 | 2384 | 2867 | 4030 | n.s. |
| Ketones | |||||||||||
| 2-Butanone | 877 | 881 | Standard | Ether-like d, fruity e | 100594 | 13176 | 2816 | 5570 | 9444 | 8845 | *** |
| 2-Pentanone | 1103 | 1023 | Literature | Fruity, wine, woody e | 318 | 9732 | 0 | 3618 | 5058 | 0 | n.s. |
| 4-Methyl-3-penten-2-one | 1112 | 1113 | Literature | Minty d | 378 | 1054 | 388 | 2804 | 1936 | 1599 | ** |
| 2-Heptanone | 1168 | 1189 | Standard | Soapc, blue cheese f | 6000 | 613 | 69 | 2061 | 712 | 6591 | *** |
| 6-Methyl-2-heptanone | 1223 | 1228 | Literature | Camphoreous e | 3796 | 547 | 190 | 1666 | 1397 | 5859 | ** |
| 2-Octanone | 1276 | 1283 | Literature | Earthy, woody, herbal, yeasty e | 2081 | 230 | 0 | 1204 | 0 | 15423 | * |
| 2,6,6-Trimetylcyclohexanone | 1304 | 1333 | Literature | Pungent d, honey, citrus f | 3322 | 641 | 414 | 3570 | 1345 | 3164 | *** |
| 6-Methyl-5-hepten-2-one | 1329 | 1339 | Standard | Mushroom, earthy, woody, rubbery d | 17970 | 3421 | 4008 | 12375 | 7464 | 26764 | *** |
| 3-Octen-2-one | 1381 | 1392 | Literature | Earthy, spicy, herbal e | 327 | 0 | 0 | 0 | 0 | 2768 | *** |
| 2-Undecanone | 1579 | 1580 | Literature | Waxy, fruity, pineapple e | 551 | 0 | 0 | 440 | 256 | 2597 | *** |
| 1-Phenylethanone | 1632 | 1645 | Literature | Almond, floral d, musty c | 6241 | 2515 | 1165 | 2683 | 2196 | 3052 | *** |
| Furans | |||||||||||
| 2-Methylfuran | 865 | 877 | Literature | Ether-like, chocolate d | 478 | 929 | 0 | 932 | 821 | 1317 | n.s. |
| 2-Pentylfuran | 1218 | 1229 | Literature | Green bean c, pungent f | 6330 | 2120 | 1877 | 31274 | 6815 | 53767 | *** |
| 5-Isopropenyl-2-methyl-2-vinyltetrahydrofuran | 1227 | 1253 | MS | Fresh, forest, grassy c | 407 | 3770 | 3579 | 25511 | 9142 | 4485 | *** |
| Furfural | 1443 | 1458 | Standard | Bread, almond c | 5093 | 379572 | 185077 | 565607 | 442585 | 380159 | *** |
| 2-Acetylfuran | 1486 | 1497 | Standard | Balsamic c | 606 | 22074 | 6933 | 35757 | 29182 | 32601 | *** |
| 5-Methyl-2-furfural | 1554 | 1560 | Standard | Almond, caramel, burnt sugar c | 1710 | 9757 | 2555 | 53826 | 23679 | 24234 | *** |
| Alcohols | |||||||||||
| 2-Methyl-1-propanol | 1089 | 1100 | Standard | Etherial, whiney e | 765 | 12669 | 497 | 619 | 4286 | 9570 | *** |
| 3-methylbutanol | 1199 | 1222 | Standard | Fusel, pungent, etherial, banana e | 50370 | 29394 | 576 | 6440 | 20607 | 33726 | *** |
| 1-Pentanol | 1248 | 1274 | Standard | Alcohol, pungent, fruity, balsamic c | 6211 | 1651 | 935 | 2987 | 2475 | 10508 | ** |
| 1-Hexanol | 1348 | 1372 | Standard | Resin, flowery, green c | 118601 | 3188 | 1663 | 0 | 3238 | 72258 | *** |
| 1-Nonanol | 1639 | 1640 | Literature | Fatty, green c | 915 | 0 | 0 | 0 | 0 | 3752 | *** |
| Phenethyl alcohol | 1894 | 1932 | Standard | Honey, spice, rose, lilac c | 0 | 3761 | 0 | 0 | 585 | 5563 | *** |
| Phenols | |||||||||||
| p-Cresol | 1884 | 1902 | Literature | Medicinal, phenol, smoke c | 2201 | 1163 | 4553 | 6744 | 6671 | 8057 | n.s. |
| Phenol | 1976 | 1987 | Literature | Phenolic, medicinal d | 6872 | 1163 | 609 | 826 | 499 | 762 | *** |
| p-Ethylguaiacol | 2005 | 2008 | Literature | Spice, clove c | 0 | 329 | 1707 | 646 | 1459 | 2854 | * |
| Acid | |||||||||||
| Pentanoic acid | 1647 | 1685 | Literature | Sweaty, pungent, sour, cheesy, beefy d | 0 | 744 | 0 | 0 | 0 | 0 | * |
| Lactone | |||||||||||
| γ-Butyrolactone | 1610 | 1617 | Literature | Caramel, sweet c | 461 | 2709 | 305 | 1072 | 1037 | 2902 | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhari, N.H.; Martens, H.J.; Petersen, M.A. Changes in Physicochemical Properties and Volatile Compounds of Roselle (Hibiscus sabdariffa L.) Calyx during Different Drying Methods. Molecules 2021, 26, 6260. https://doi.org/10.3390/molecules26206260
Juhari NH, Martens HJ, Petersen MA. Changes in Physicochemical Properties and Volatile Compounds of Roselle (Hibiscus sabdariffa L.) Calyx during Different Drying Methods. Molecules. 2021; 26(20):6260. https://doi.org/10.3390/molecules26206260
Chicago/Turabian StyleJuhari, Nurul Hanisah, Helle Jakobe Martens, and Mikael Agerlin Petersen. 2021. "Changes in Physicochemical Properties and Volatile Compounds of Roselle (Hibiscus sabdariffa L.) Calyx during Different Drying Methods" Molecules 26, no. 20: 6260. https://doi.org/10.3390/molecules26206260