Abstract
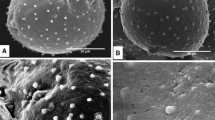
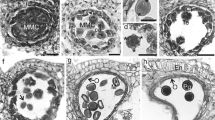
Pollen grains of three Brazilian species of Passiflora (P. elegans, P. suberosa and P. haematostigma) belonging to different subgenera were studied with respect to the wall and cytoplasm. New data were obtained on pollen wall histochemistry, cytoplasm contents and organelle inheritance. The structure of the pollen wall layers differed in all the species; P. elegans shares characters with those found in other species from the same subgenus. The exine foot layer is structured and evident only in P. haematostigma and is not structured in P. elegans. The pollen grains have pollenkitt with lipid components. The cytoplasm of the vegetative cell contains dissolved and non-dissolved polysaccharides. The generative cell contains plastids and mitochondria in all the species analyzed, and consequently has the potential for paternal or biparental extranuclear inheritance. Aspects of the evolution of the characters of the species are discussed in the light of a recent phylogeny of the group, with a focus on the three subgenera.






Similar content being viewed by others
References
Acioli MF (1999) Estudo Preliminar da Biologia Floral de Passiflora suberosa Linnaeus (Passifloraceae). Monografia (Trabalho de conclusão do curso de Bacharelado). Instituto de Biociências. Universidade Federal do Rio Grande do Sul, Porto Alegre
Acioli MF (2003) Ecologia da Polinização de Passiflora suberosa Linnaeus (Passifloraceae). Dissertação de Mestrado. Instituto de Biociências. Universidade Federal do Rio Grande do Sul, Porto Alegre
Amela Garcia MT, Hoc PS (1998) Biologia Floral de Passiflora foetida (Passifloraceae). Rev Biol Trop 46(2):191–202
Amela Garcia MT, Galati BG, Anton AM (2002) Microsporogenesis, microgametogenesis and pollen morphology of Passiflora spp. (Passifloraceae). Bot J Linn Soc 139:383–394
Angiosperm Phylogeny Group (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161:105–121
Baker HG, Baker I (1979) Starch in angiosperm pollen grains and its evolutionary significance. Am J Bot 66(5):591–600
Bozzola JJ, Russel LD (1998) Electron microscopy: principles and techniques for biologists. Jones and Bartlett, Boston
Braum AF (2008) Morfologia, anatomia e imunocitoquímica da interação entre pólen e estigma em duas espécies de Passiflora (Passifloraceae). Dissertação de Mestrado. Universidade Federal do Rio Grande do Sul, Porto Alegre
Bray DF, Wagenaar EB (1978) A double staining technique for improved contrast of thin sections from Spurr-embedded tissue. Can J Botany 56:129–132
Carreira LMM (1977) Aspectos da ultra-estrutura do pólen de Passiflora coccinea Aubl. (Passifloraceae). Acta Amazonica 7(3):329–332
Corbet SA, Beament J, Eisikowitch D (1982) Are electrostatic forces involved in pollen transfer? Plant Cell Environ 5:125–129
Corriveau JL, Coleman AW (1988) Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am J Bot 75(10):1443–1458
Cronquist A (1988) The evolution and classification of flowering plants, 2nd edn. Bronx, New York
Dettke GA, Santos RP (2011) Morfologia externa, anatomia e histoquímica da antera e grãos de pólen de Passifloraceae do Rio Grande do Sul, Brasil. Rev Bras Bioc 9(s1):48–74
Erdtman G (1952) Pollen morphology and plant taxonomy: angiosperms. The Chronica Botanica Co, Waltham
Erdtman G (1960) The acetolysis method. A revised description. Sven Bot Tidsskr 54:561–564
Feder N, O′Brien TP (1968) Plant microtechnique, some principles and new methods. Am J Bot 55:123–142
Gerrits PO, Smid L (1983) A new, less toxic polymerisation system for the embedding of soft tissue in glycol methacrylate and subsequent preparing of serial sections. J Microsc-Oxford 132:81–85
Hanaichi T, Sato T, Iwamoto T, Malavasiyamashiro J, Hoshiro M, Mizuno N (1986) A stable lead by modification of Sato method. J Electron Microsc 35(3):304–306
Hansen AK, Gilbert LE, Simpson BB, Downie SR, Cervi AC, Jansen RK (2006) Phylogenetic Relationships and Chromosome Number Evolution in Passiflora. Syst Bot 31(1):138–150
Hansen AK, Escobar LK, Gilbert LE, Jansen RK (2007) Paternal, maternal and biparental inheritance of the chloroplast genome in Passiflora (Passifloraceae): implications for phylogenetic studies. Am J Bot 94(1):42–46
Heslop-Harrison J, Heslop-Harrison Y (1991) Structural and functional variation in pollen intines. In: Blackmore S, Barnes SH (eds) Pollen and spores: patterns of diversification. Clarendon Press, Oxford
Hesse M (2000) Pollen wall stratification and pollination. Plant Syst Evol 222:1–17
Huang CY, Ayliffe MA, Timmis JN (2003) Direct measurement of the transfer rate of chloroplast DNA into the nucleus. Nature 442:72–76
Ji X, Zhang Q, Liu Y, Sodmergen (2004) Presence of plastid and absence of mitochondrial DNA in male reproductive cells as evidence for cytoplasmic inheritance in Turnera and Zantedeschia aethiopica. Protoplasma 224:211–216
Knox RB (1984) The pollen grain. In: Johri BM (ed) Embryology of angiosperms. Spring-Verlag, Berlin
Koschnitzke C, Sazima M (1997) Biologia Floral de cinco espécies de Passiflora L. (Passifloraceae) em mata semidecídua. Rev Bras Bot 20:119–126
Knox RB, Heslop-Harrison J (1970) Pollen-wall proteins: localizations and enzymatic activity. J Cell Sci 6:1–27
Krosnick SE, Freudenstein JV (2005) Monophyly and floral character homology of old world Passiflora (Subgenus Decaloba: supersection Disemma). Syst Bot 30(1):139–152
Larson DA (1966) On the significance of the detailed structure of Passiflora caerulea exines. Bot Gaz 127:40–48
Lillie RD (1965) Histopathologic technic and practical histochemistry. McGrawHill, New York
MacDougal J, Feuillet C (2004) Systematics. In: Ulmer T, MacDougal JM (eds) Passiflora: passionflowers of the world. Timber Press, Portland, pp 27–31
Martin FW (1959) Staining and observing pollen tubes in the style by means of fluorescence. Stain Technol 34:125–128
Mogensen HL (1996) The hows and whys of cytoplasmic inheritance in seed plants. Am J Bot 83:383–404
Muschner VC, Lorenz-Lemke AP, Vecchia AM, Bonatto SL, Salzano FM, Freitas LB (2006) Differential organellar inheritance in Passiflora’s (Passifloraceae) subgenera. Genetica 128:449–453
Muschner VC (2005) Filogenia Molecular, taxas evolutivas, tempo de divergência e herança organelar em Passiflora L. (Passifloraceae). Tese de Doutorado. Universidade Federal do Rio Grande do Sul, Porto Alegre
Muschner VC, Lorenz AP, Cervi AC, Bonatto SL, Souza-Chies TT, Salzano FM, Freitas LB (2003) A first molecular phylogenetic analysis of Passiflora (Passifloraceae). Am J Bot 90(8):1229–1238
Nepi M, Franchi GG (2000) Cytochemistry of mature angiosperm pollen. Plant Syst Evol 222(1–4):45–62
O’Brien TP, McCully ME (1981) The study of plant structure: principles and selected methods. Termacarphi Pty Ltd, Melbourne
Pacini E (2000) From anther and pollen ripening to pollen presentation. Plant Syst Evol 222:12–43
Pacini E, Franchi GG, Ripaccioli M (1999) Ripe structure and histochemistry of some gymnosperms. Plant Syst Evol 217:81–99
Pacini E, Hesse M (2005) Pollenkitt - its composition, forms and functions. Flora 200:399–415
Presting D (1965) Zur Morphologie der Pollenkörner der Passifloraceen. Pollen Spores 7:193–245
Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A (2007) Glossary of pollen and spore terminology. Rev Paleobot Palyno 143(1–2):1–81
Rêgo MMR, Bruckner CH, Silva EAM, Finger FL, Siqueira DL, Fernandes AA (1999) Self-incompatibility in passion fruit: evidence of two locus genetic control. Theor Appl Genet 98(3–4):564–568
Rêgo MM, Rêgo ER, Bruckner CH, Da Silva EAM, Finger FL, Pereira KJC (2000) Pollen tube behavior in yellow passion fruit following compatible and incompatible crosses. Theor Appl Genet 101(5–6):685–689
Roland JC, Vian B (1991) General preparation and staining of thin sections. In: Hall JL, Hawes C (eds) Electron microscopy of plant cells. Academic Press, London
Russell SD (2003) Plant sexuality, cell expression and preferential fertilization. Bol Soc Argent Bot 38(3–4):349–356
Sass JE (1951) Botanical microtechnique, 2a edn. The Iowa State College Press, Ames
Sazima M, Sazima I (1978) Bat pollination of the passion flower. Passiflora mucronata in southeastern Brazil. Biotropica 10:100–109
Sears BB (1980) Elimination of plastids during spermatogenesis and fertilization in the plant kingdom. Plasmid 4:233–255
Shore JS, McQueen KL, Little SH (1994) Inheritance of plastid DNA in the Turnera ulmifolia complex (Turneraceae). Am J Bot 81:1636–1639
Shore JS, Triassi M (1998) Paternally based cpDNA inheritance in Turnera ulmifolia (Turneraceae). Am J Bot 85:328–332
Skvarla JJ, Larson DA (1966) Fine structure studies of Zea mays pollen. I. Cell membranes and exine ontogeny. Am J Bot 53(10):1112–1125
Southworth D (1973) Cytochemical reactivity of pollen walls. J Histochem Cytochem 21(1):73–80
Suassuna TMF, Bruckner CH, Carvalho CR, Borém A (2003) Self-incompatibility in passionfruit: evidence of gametophytic–sporophytic control. Theor Appl Genet 106:298–302
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultra Mol Struct R 26:31–34
Timmis JM, Ayliffe M, Huang CY, Martin W (2004) Endosymbiotic gene transfer organelle genomes forge eukaryotic chromosomes. Nat Rev Genet 5:123–135
Ulmer T, MacDougal JM (2004) Passiflora. Passionflowers of the world. Timber Press, Portland
Varassin IG, Trigo JR, Sazima M (2001) The role of nectar production, flower pigments and odour in the pollination of four species of Passiflora (Passifloraceae) in south-eastern Brazil. Bot J Linn Soc 136:139–152
Weber M (1992) The formation of pollenkitt in Apium nodiflorum (Apiaceae). Ann Bot 70:573–577
Whatley JM (1982) Ultrastructure of plastid inheritance: green algae to angiosperms. Biol Rev 57(4):527–569
Zhang Q, Yang L, Sodmergen (2003) Examination of the cytoplasmic DNA in male reproductive cells to determine the potential for cytoplasmic inheritance in 295 Angiosperm species. Plant Cell Physiol 44(9):941–951
Acknowledgments
We thank Armando Carlos Cervi and Greta Aline Dettke for collecting P. haematostigma in the field, and the Centro de Microscopia Eletrônica (CME-UFRGS) for making the equipment available. This article is part of the PhD thesis of the first author, carried out with the support of a CAPES doctoral scholarship. The project was supported by CNPq-Proc. 305289/2008-0, 480622/2007-8, 474132/2003-4 and 300822/2005-7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silvério, A., de Araujo Mariath, J.E. Comparative structure of the pollen in species of Passiflora: insights from the pollen wall and cytoplasm contents. Plant Syst Evol 300, 347–358 (2014). https://doi.org/10.1007/s00606-013-0887-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-013-0887-6